Aluminum offers a lighter weight and lower cost than copper so its at first glance it may seem like a great way to replace more expensive copper wiring. If χis negative then the material is diamagnetic and the magnetic field is weakened in the presence.

How Many Valence Electrons Does Aluminum Al Have
Plastics are entirely different.

. In the case of your remote the device is. Unlike pure copper wire copper-clad aluminum uses an aluminum wire core with a thin copper plating. In my own testing of A123 18650 LifePO4 cells charged to 80 and stored for 1 years they only lost about 25 of their charge 2month.
Compared to high quality Li-Ion cobalt 18650 cells charged to 50 and stored. When it forms an Al 3 ion it loses the 3-level electrons to leave 1s 2 2s 2 2p 6. For example chemical engineers study the reactions responsible for producing charged particles to create electricity.
Aluminum Copper Diamond Tungsten Hydrogen 1 atm Oxygen 1 atm Nitrogen 1 atm Susceptibility of diamagnetic and paramagnetic materials If χ is positive then the material is called paramagnetic and the magnetic field is strengthened by the presence of the material. Davy published a chemistry textbook in which he used the spelling aluminum. Aluminum is the primary spelling in the United States and Canada while aluminium is in the rest.
Orbiting around the nucleus are rings or shells of electrons. Many fields of engineering require that people have a good understanding of electricity. Its monatomic form H is the most abundant chemical substance in the Universe constituting.
Most batteries have two easily identifiable poles a positive and a negative. Material engineers make many substances that serve as conductors and insulators. Atomic size decreases from.
Both spellings have coexisted since. The electrons have a negative electrical charge. However their usage has split by region.
Electrical engineers are able to control electricity by changing the. From the outside it misleadingly looks the same because of the plating. The neutron is a subatomic particle symbol n or n 0 which has a neutral not positive or negative charge and a mass slightly greater than that of a protonProtons and neutrons constitute the nuclei of atomsSince protons and neutrons behave similarly within the nucleus and each has a mass of approximately one atomic mass unit they are both referred to as nucleons.
When the battery isnt in use electrons collect at the negative pole. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions see below. Metals generally have low resistance because electrons can flow through them quite easily.
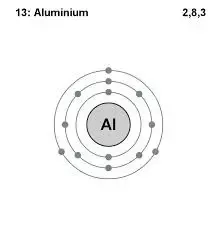
The electron configuration of aluminum is 1s 2 2s 2 2p 6 3s 2 3p x 1. - Covalent compounds are formed by sharing electrons between atoms - Most of the compounds that we come in contact with are covalent compounds - Covalent compounds contain covalent bonds - Nitrogen N2 is a covalent compound - Covalent compounds are formed by transferring electrons from one atom to another atom. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
When the batteries are inserted into a device the two poles come into contact with the sensors in the device closing the circuit and allowing electrons to flow freely between the poles. There are many references on the web that state LifePO4 and conventional Li-Ion cobalt BOTH have typical self discharge rates of 5 or less per month. The aluminum reorganizes hybridizes six of these the 3s three 3p and two 3d to produce six new orbitals all with the same energy.
Atomic size measured the distance between the nucleus of an atom and the outermost non-valence electrons of the atom. Neutrons are of course neutral. Periodic trends predict differences between elemental characteristics as you move across the periodic table.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The harder it is for electrons to flow the more resistance there is. The number of protons in an atom is its atomic number.
The protons have a positive electrical charge. Trends are based on Coulombs law which mathematically relates several characteristics of an elements. Electrons have to flow through a material to carry electricity through it.
Three main reasons for the increase in photocatalytic activity of Bi2O3SrFe12O19 were 1 the formation of p-n-type heterojunction between p-type Bi2O3 and n-type SrFe12O19 semiconductors 2 magnetic field effect stemming from magnetic composite itself generated a shunt effect for photoexcited electrons and 3 as a sensitizer absorbing visible light. There are as many electrons as there are protons so the atom is balanced electrically or neutral. Although often solid they dont have the same crystalline structure.
That means that all the 3-level orbitals are now empty.

How Many Valence Electrons Does Aluminum Al Have
How Many Valence Electrons Are In Aluminium Quora

0 Comments